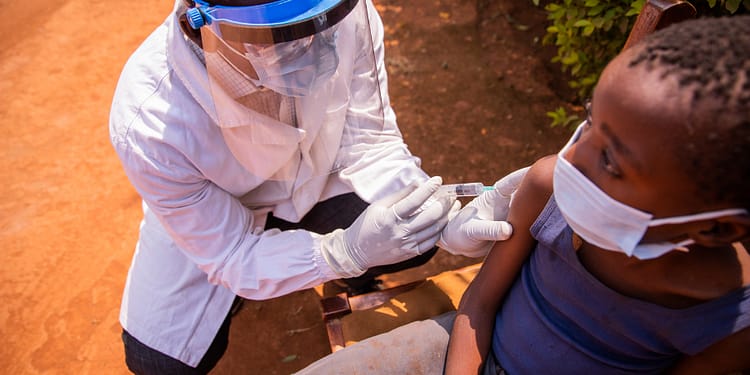
Uganda has started a Pioneering clinical efficacy trial of Sudanese Ebola vaccine. This trial is the first of its kind and is being conducted with unprecedented speed. With the support of the World Health Organization (WHO) and several international allies, this initiative will evaluate a vaccine that could change the way the virus is fought.
An unprecedented achievement in times of emergency
The Ugandan Ministry of Health, WHO and other partners have worked tirelessly to launch this trial in just four days. It was made possible through international collaboration, which has ensured that the trial meets ethical and regulatory requirements.
This is a randomized ring vaccination trial. It has been designed to evaluate the efficacy of a vaccine that has already shown safety and immunogenicity in previous trials. A single dose of the candidate vaccine will be administered to close contacts and contacts of contacts of a confirmed Ebola case.
This is the first vaccination trial conducted in the context of an active outbreak of the Ebola virus, caused by the Sudanese species. Although several vaccines are in development, there are no authorisations for the use of any vaccine against this specific variant of the virus.
Global collaboration for pandemic preparedness
The trial is made possible through the joint efforts of several organizations. IAVI donated the vaccine with financial support from WHO, the Coalition for Epidemic Preparedness Innovations (CEPI), Canada’s International Development Research Centre (IDRC), and the European Commission’s Health Emergency Preparedness and Response Authority (HERA). In addition, the Africa Centers for Disease Control and Prevention (Africa CDC) provided additional support.
WHO Director-General Dr Tedros Adhanom Ghebreyesus stressed that this progress is crucial for pandemic preparedness. He underlined the importance of local and international collaboration, and thanked Uganda’s health workers and all partners for their dedication. “This colossal achievement would not have been possible without all of them,” he said.
An essay that gives hope in the face of the outbreak
The ring vaccination trial is based on a strategy that has been used successfully in previous outbreaks. It involves vaccinating people who have been in close contact with a confirmed case, creating a small immunization zone that can limit the spread of the virus.
If the vaccine proves effective, it could play a key role in containing this outbreak and provide valuable data for future vaccine authorization. The study follows the protocol used in previous trials, such as the one conducted in Guinea in 2015.
The progress of this trial is crucial to improving the response to future Sudanese Ebola outbreaks. As it moves forward, the focus remains on the safety of participants and the validity of the data obtained, which could represent a significant change in the fight against Ebola.
A step towards a safer future
This trial is the result of years of preparation and cooperation. Training in clinical research practices and standard operating procedures was conducted, ensuring that the trial is conducted in a rigorous manner. The vaccine doses were previously stored in Uganda, and WHO was responsible for ensuring that all storage standards were met.
With this trial, Uganda and the international community hope not only to curb the current outbreak, but also to lay the groundwork for developing new tools against Ebola in the future.